
Astronomical Spectra
Investigating the Spectra of Astronomical Phenomena
When two hydrogen atoms fuse together they create energy during a nuclear fusion reaction, which powers stars like the Sun. The reaction converts mass to energy, so the amount of energy created is immense. As well as heat energy, the fusion reaction also produces white light.
In the late 17th Century, Isaac Newton experimented with sunlight and a prism, having noticed how the light produced the colours of the rainbow. He declared that the visible light from the Sun was made up of a composite of seven separate colours which combined to produce white light. He came up with an explanation for the phenomena, which was that light is made up of "corpuscles", in other word discrete particles of light. This explanation was innovative but it had many flaws and various astronomers and physicists (notably Christiaan Huygens and Albert Einstein) tried to resolve the problems of Newton's particle theory of light which has led us, three hundred years after Newton, to the photon model in which all electromagnetic radiation, including light, is made up of discrete packets of energy that vibrate and travel at the speed of light in a vaccuum. It is the frequency of vibration that gives rise to the different phenomena such as X rays, radio waves and visible light. The range of wavelengths (hence frequencies) gives rise to the electromagnetic spectrum described in the previous section.
In 1814, Joseph von Fraunhofer, a German physicist and optical lens manufacturer, observed bright lines at certain wavelengths when the light of a flame was passed through his apparatus, which included a diffraction grating that he had invented. A diffraction grating is a flat sheet of glass with extremely fine lines etched onto the surface, which diffracts light and acts like a prism. He also noticed dark bands when the light from a star was passed through his diffraction grating. The consistency of the positioning of bright lines from flames produced by burning different materials, and the dark bands in the spectra from stellar light led him to conclude that the dark and light bands were somehow related and had something to do with the material that made up the stars.
It was found that the dark bands in the spectrum corresponded to very specific wavelengths which also corresponded to the light bands produced by burning different materials. The flame of burning material gives off light at specific wavelengths , but when white light passes through a gas made of the same material, the material actually absorbs energy at those wavelengths, creating a dark line in the spectrum. These lines are known as Fraunhofer lines. This was a monumental discovery: by comparing the dark lines in a spectrum with those produced by known elements, the constituent atoms of distant astronomical phenomena could be derived.
In summary, there are three different types of spectrum that are used to explain Fraunhofer's discovery: Continuous, Emission and Absorption Spectra. A useful illustration of this can be found below and on the James Webb Telescope website (for URL see panel).
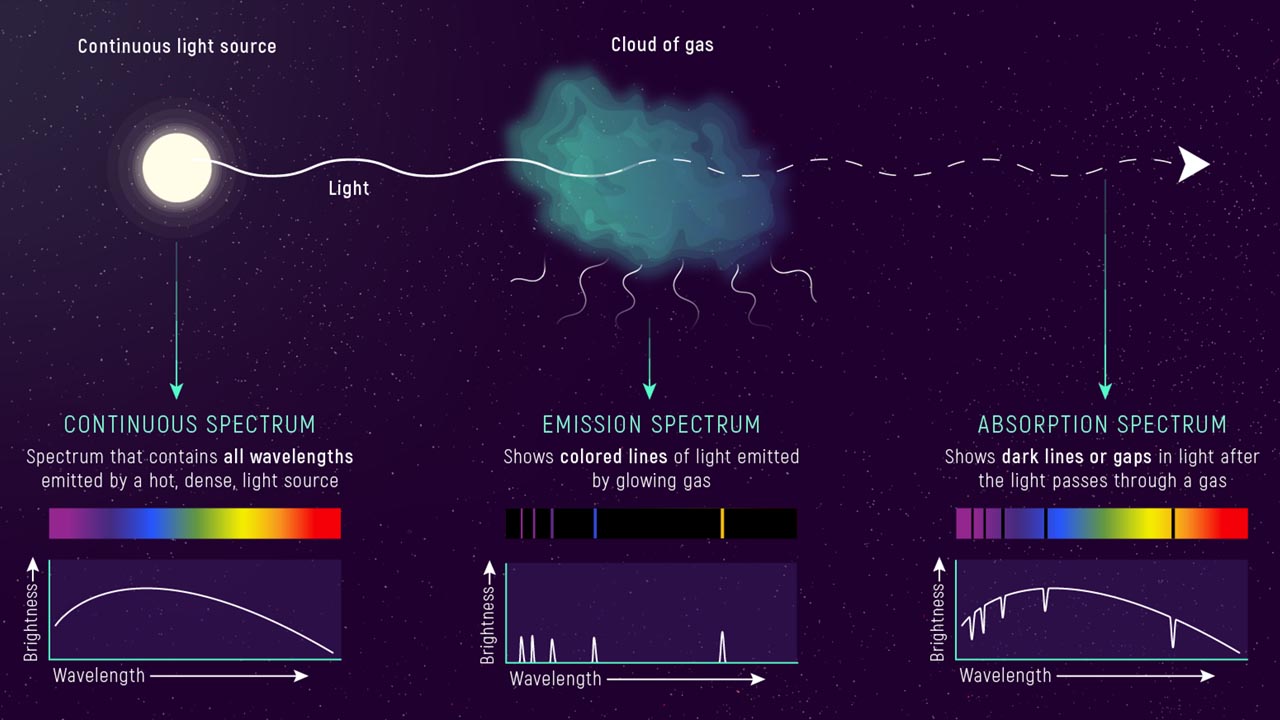
Continuous Spectrum
Light from a hot object such as a star gives off light that shows a continuous spectrum, in other words there are components of light at all frequencies. Hotter stars emit more from the blue end of the spectrum whilst cooler stars emit more light from the red end of the spectrum.
Emission Spectrum
When the light from a continuous spectrum source passes through a cloud or gas of atoms, those atoms get excited and emit light but this time at very specific wavelengths, and scattered in different directions. This gives rise to the bright lines in the spectrum.
Absorption Spectrum
When the light from a continuous emission source passes through a cloud or gas of atoms, the beam of light viewed end on is altered. The atoms of the cloud absorb photon energy at specific wavelengths and as the photons are removed from the light beam this gives way to dark bands in the spectrum. The image above shows that within the same cloud of atoms, the bright lines of emission spectra correspond to the black lines of absorption spectra.
In the projects section the phenomena of absorption spectra are utilised in a practical demonstration of astronomical spectroscopy.